Document Type : Review Article
Authors
1 Chemistry Department, College of Science, University of Raparin, Al Sulaymaniah, 46012 Ranya, Iraq
2 Physics Department, College of Science, University of Halabja, 46018, Halabja, Iraq
Abstract
Phthalocyanines (Pcs) are macrocyclic chemical compounds that have attracted a lot of attention in the last decade due to their varied properties. Over time, the extensive utilization of PCs has been justified due to their exceptional nature, with their numerous desirable qualities stemming from the straightforward and adaptable methods for altering their structure. These modifications have enabled a wide range of applications, including photodynamic therapy for cancer treatment, chemical sensing, solar cells, nonlinear optics, and, most recently, photoinitiator systems for various polymerization processes, including free radical, cationic, and controlled radical polymerizations. Pcs distinctive electrochemical, photochemical, and photophysical properties have made all of these advancements possible. Our motivation to emphasize the significance of phthalocyanines in chemical science is driven by their remarkable success story. This study commences with an exploration of the design and synthesis of Pcs tailored for specific applications, followed by a spotlight on innovative research that harnesses the diverse activation capabilities of these macrocycles to initiate various types and also describes characterization.
Graphical Abstract
Keywords
Main Subjects
- Introduction
oordination chemistry is a branch of chemistry that focuses on the study of coordination compounds. It involves the examination of compounds in which a central metal atom is encompassed by one or more anions and molecules, with potential direct connections to the metal. These connections may or may not result in a charge within the complex. When a complex carries an electrical charge, its stability is maintained by counter-ions in its immediate vicinity. The central metal ions serve as the central structural elements of these complexes, and they are surrounded by a diverse assortment of neutral molecules and/or ions. While it is tempting to assume that coordinating covalent bonds solely link these elements to the central ion, the actual bonding in coordination compounds is typically more intricate. The molecules or ions forming the outer layer around the metal ion are referred to as ligands [1].
Phthalocyanines are indeed part of coordination chemistry. They are a class of coordination compounds that consist of a central metal atom or ion coordinated with phthalocyanine ligands. In this context, they are a specific example of compounds studied within coordination chemistry, where the focus is on understanding the interactions and properties of such coordination complexes [2].
Phthalocyanines (Pcs) are organic or organometallic compounds owning the basic porphyrin ring structure of formula (C32H18N8), shown in Scheme 1 [3]. Phthalocyanines are planar and macro-heterocyclic compounds [4,5] and solid at room temperature [6]. The molecule of Pcs has an aromatic structure and possesses exceptional stability due to its conjugation. Pcs are macrocycle compounds having an 18π-electrons system [7,8]. Pcs compounds are obtained from about seventy different metals and could have been prepared [4].

Scheme 1. Phthalocyanine structure (1)
The chemical formula for phthalocyanine is 29H, 31H- tetrabenzo [b,g,l,q]- 5, 10, 15, 20-tetraazaporphine. According to the Hückel rule (4n+2), when n = 10, the dianionic ligand with 42 electrons displays pronounced aromaticity, as displayed in Scheme 2 [9], as well as center metal atoms [10].

Scheme 2. Metal phthalocynine structure (2)
Phthalocyanines, unearthed during the early 19th century, are one of the few types of colorants still in use today, The discovery and synthesized of phthalocyanines was a complete accidentally, also in 1907, Von Braun produced a phthalocyanine that included no metals [11], and de Diesbach prepared Cu-Pcs in 1927 [12]. Although they failed to define their findings, chemists at Scottish Dyes Ltd are credited with discovering phthalocyanines when they synthesized and analyzed Fe- Pcs. According to the generally accepted account of their discovery, four chemists named Dandridge, Drescher, Dunworth, and Thomas came upon an insoluble blue substance during the regular production of phthalimide from phthalic anhydride, both of which are white and solids. A registered application was filed in 1928 [13]. The product, which was collected as phthalimide, was discovered to be tainted with a dark-colored impurity. The impurity remained in the Phthalimide despite heating or dissolving in the water and most reagents [14]. Phthalimide structure is shown in Scheme 3. This compound was created by reacting phthalic anhydride with ammonia in a glass-lined iron kettle [15]
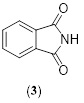
Scheme 3. Phthalinide structure (3)
The phthalimide and impurity were separated and examined [16]. When cuprous chloride was introduced into molten phthalimide, a vigorous reaction occurred, resulting in the formation of a colored product [17]. The metals had to be a key part of the structure of this new compound. This structure was later confirmed by X-ray diagrams [18].
The name of "phthalocyanine" contained from the Greek words for "rock oil" (naphtha) and "dark blue" (cyanine). This term was originally coined by Sir Reginald Linstead in 1934 to describe these macrocyclic molecules [19].
However, Pc is the first name of a particular compound that represents substituted and unsubstituted Pcs [20].
In 1934, Linstead and his colleagues conducted experiments that clarified the structure of metal- Pc [21,22]. Many different physical features of Pc were studied between the 1930s and the 1950s, including its X-ray spectrum, reduction, oxidation, and absorption spectra, in other words, Pcs possess various attributes such as their ability to act as catalysts, exhibit magnetic characteristics, and numerous other properties. As a result, Pcs can be described as flat aromatic ring compounds composed of 18-electron atoms and displaying a wide range of colors, reminiscent of porphyrins [23,24]. Prior to 1960, there existed highly conjugated phthalocyanines were already in existence, and these derivatives of Pcs exhibited a robust structural framework. Their valuable chemical and physical characteristics, such as vibrant colors and conductivity, along with their exceptional chemical and thermal stability, gave them a considerable edge. This led to their extensive adoption in a variety of applications [25].
Metal phthalocyanines (M-Pcs) are identified with over 70 distinct elemental ions, including hydrogen without metal phthalocyanine (H2-Pc) [26]. The molecules have been given bonding between the center atom (its metals) with a great organic ring structure surrounding it. The central metal atom is surrounded by four nitrogen atoms like a square. It has two kinds of bonding possibly covalent and electrovalent bond [27]. Besides these have found widespread use in electronics, electrophotography, solar cells, liquid crystals, chemical sensors, and Langmuir-Blodgett films [28,29]. When the core metal or the pivot or permutation rings of phthalocyanines are altered, they find applications in a wide range of disciplines [30]. Metal-free organic sensitizers can shift the Soret and Q bands toward lower energy absorption by employing porphyrins and phthalocyanines compounds, while others use strong electron donating or withdrawing moieties to tune the optical band gap [31].
1.1 Typical properties of phthalocyanines
Pcs are thermally stable, resistant to strong acids and bases [6], that have been effectively used as working parts in semiconductor and electrochromic gadgets [32]. However, phthalocyanine has low solubility and limited solubility in DMSO and other polar chemical solvents [33].
Pcs exhibit high absorption in the red (700 nm) portion of the visible spectrum [34]. Pcs have great promise as photosensitizers for use in photodynamic therapy (PDT) of cancer [35,36], they are ideal candidates for PDT because of their numerous benefits [37,38]. However, they have been used successfully implemented as active components in semiconductor and electrochromic devices [32].
Purification of substituted Pcs might be accomplished by soluble in strong sulphuric acid, then precipitating in cold water or ice, for amino-substituted Pcs, dissolving in concentrated hydrochloric acid, then precipitating in water. Therefore, alumina column chromatography with evaporation or recrystallization of the solvent and conventional, flash, or vacuum column chromatography on silica gel, then the solvent is evaporated or the crystals are reformed [23,39]. Gel chromatography can separate molecules based on their size, but it cannot differentiate mononuclear Pcs from those in folded conformations. Washing with solvents can remove very insoluble substituted Pcs from soluble impurities, but this process leaves other insoluble impurities behind [40], as shown in Scheme 4.

Scheme 4. Reagents and conditions for MPc synthesis (2): i. Mix metal salts with a solvent that has a high boiling point (hydroquinone). ii. Mix the urea and metal salt in a solvent with a high boiling point. Iii. Lithium, then pentanol aqueous hydrolysis at high temperature. iv. Warm a metal salt dissolved in alcohol.
- Synthesis of Phthalocyanines
Metal-substituted or non-substituted phthalocyanines might be produced that have not been replaced and can be employed as a stable colorant [41]. These issues are solved by phthalocyanine, which includes dendrimers and polymers. Aside from that, Phthalocyanine is made up of polymers with different names depending on their side groups and the main chain of the polymeric cycle. Organic solvents do not dissolve polymerics or macrocyclic phthalocyanines. It takes a lot of effort to achieve utility and Efficiency [42].
Phthalocyanines may exhibit varying properties in both their solid and solution phases, particularly in terms of nonlinear optical properties. The excitonic effect, which is linked to intermolecular interactions, might explain this discrepancy [43].
2.1. Synthesis of hydrogen substituted phthalocyanines
As demonstrated in Scheme 5, unsubstituted H2Pc may be made from phthalonitrile (1) or 1,3-diiminoisoindoline (5). In most organic solvents, the molecule is almost insoluble. In high boiling solvents like 1 -chloronaphthalene, quinoline, and others, it can only be minimally dissolved. The most frequent technique for forming H2Pc is (4) (1,2-dicyanobenzene), which may be done in various ways. (1) reacts with ammonia to generate (5) (1,2- diiminoisoindoline), which then reacts in a milder condition to form H2Pc [44].

Scheme 5. Regaents and conditions for H2Pc synthesis: i. Lithium, followed by aqueous hydrolysis of boiling pentanol. Ii. Add hydroquinone to the mix. iii. Heat in a pentanol solution containing DBN/DBU. v. Reflux of alcohol with a high boiling point. iv. Ammonia, boiling methanol, sodium methoxide
One example of a reducing agent is 1,8-diazobicyclo(4.3.0)non-5-ene (DBN), which may be used as a melt or a pentanol solution, with free ion and metal, is used in the cyclotetramerization procedure, with the result as M-Pc impurities [45, 39].
Many papers on the synthesis and research of heterocyclic substituted metal phthalocyanine have been published in recent years [46]. The physical and chemical characteristics of the Pc macrocycle are greatly influenced by the substituents and metal atoms introduced, allowing it to be used in a variety of applications [47].
Metal halides (MXn) or/and phthalonitrile (PN), phthalic anhydride (PA), and phthalimide (PI) were used to optimize the reaction conditions. The CuCl: phthalonitrile: hexamethyldisilazane (HMDS): dimethyl formamide (DMF): para toluene sulphonic acid (p-TsOH.H2O) molar ratio of 1:4:4:1:0.41 produced the greatest results. In a typical experiment, PN, PA, or PI was mixed with HMDS, Metal halides (MXn), p-TsOH, and DMF in a glass tube and swirled for 20 minutes [45], refluxing with more metal solution [23], under a nitrogen environment. After that, the tube was sealed and the mixture was microwaved for 10-15 minutes. To further investigate the breadth and limits of this reaction, we used various raw materials and metal salts, including Cu(OAc)2.H2O, CuCl2.2H2O, CoCl2, NiCl2, FeCl2.4H2O, ZnCl2, PdCl2, PtCl4, and RuCl3, as can be seen from Table 1 [45].
Table 1. MPc synthesis by the reaction of PN, PI, or PA under microwave irradiation [49]

After several minutes, various starting materials such as PN, PA, and PI are transformed into MPcs with good yields. It's worth noting that in the absence of HMDS and p-TsOH.H2O as a catalyst, the reaction yields plummeted, and in the case of the PN, the process was totally halted. Furthermore, no reaction has happened with any of the raw ingredients in the absence of DMF [45]. The Pcs with various central metals and peripheral substituents were found to have heavier central metals [48] , as showed in Scheme 6.
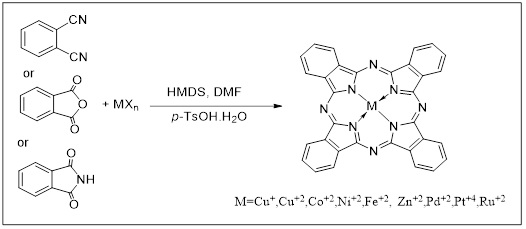
Scheme 6. Microwave-irritated hexamethyl dislazane to create phthalocyanines
2.2. Synthesis of peripheral substitution and fused ring (Mixed and cross-condensations)
M-Pc analogs and related systems [50] have been made, going back to Linstead and co-workers' investigations in the 1930s, by condensation operations employing various methodologies to make compounds having AAAA, AAAB, AABB, ABAB, ABBB, and BBBB structures. In this review, we use Leznoff's phrase [51], which refers to the total separation of the various structures [52,53]. Prepare the replies that have been well reviewed [54].
There are four items available [55]. Even so, Recent research on low symmetry Pc has centered on the readily distinguishable AAAB product produced by porphyrazine (Pz), naphthalocyanine (Nc), unsubstituted Pc, R-substituted Pc, [56] instead of tetrapyrazo porphyrazine,-substituted Pc, [57] and R,- instead of Pc [58] AAAA parent chemical substance, as well as Pc, a partially fused Pc,[59] R-substituted Pc [60] Pc AAAA parent compounds with R,-substituted [61], as shown in Scheme 7.
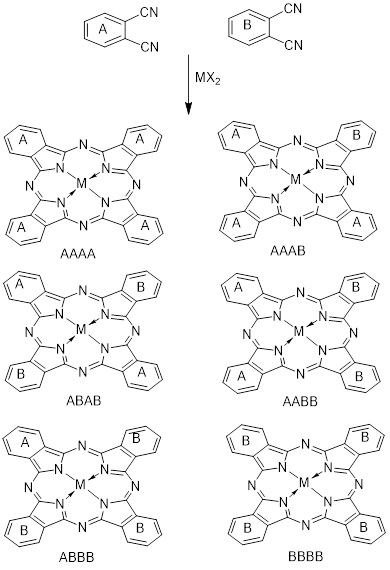
Scheme 7. Concentration of twice PNS, A and B to yield the following products
Since the late 1980s, various selective techniques for the synthesis of particular low-symmetry Pc have been established [62]. The production of poor symmetry free base AAAB complexes was highlighted by Kobayashi et al. in 1990 [63]. Hoffman and Barrett used this strategy to a limited extent [64]. In recent years, Several selective techniques for the synthesis of targeted low symmetry Pc compounds have been developed since the late 1980s [62,65].
2.3 Phthalocyanine-porpherene linkage synthesis
The Sonogashira cross coupling reaction proved effective in creating more plural structural systems of Pc with sub Pc [66]. The electron transport mechanisms in highly conjugated systems were then explored. In addition, NH-linked Zn-Pc was prepared [67]. In the presence of potassium tert-butoxide, Under the Buchwald-Hartwig amination conditions utilizing Pd(OAc)/rac-BINAP as the catalytic system, the interaction between amino-functionalized porphyrin 8 and 4-iodophthalonitrile resulted in the formation of interconnected entities. These entities were linked to the meso-phenyl group [67], as shown in Scheme 8.

Scheme 8. Construction of Pc-porphyrins with an NH-linkage with a meso-phenyl group replaced
2.4 Double-deckers phthalocyanines synthesis
Lutetium (C30H28N30)4 [Double Deakers] The Z-scan process was used to produce 2Lu. From the nonlinear absorber 4-Nitro phthalonitrile, this product might be obtained by a base-catalyzed nucleophilic nitro group displacement method. To make 4-[2-(2H-1,2,3-Benzotriazol-2-yl)-4,6-bis(2-phenylpropan-2-yl) phenoxy] phthalonitrile, a single-step reaction was performed at 70-90 °C in dry DMF in an argon atmosphere. 5-[2-(2H-1,2-Benzotriazol-2-yl)-4,6-bis(2-phenylpropan-2-yl) phenoxy] To get bis(phthalocyaninato) lutetium(III) complex, 1,3-diiminoisoindoline was reacted with lutetium(III) acetate monohydrate in octanol under the catalytic influence of DBU for 12 hours at high temperature in an argon atmosphere. Next, a precipitate (250 mL) was made by dissolving the mixture in water. The solution was diluted to a volume of 250 mL with water and allowed to precipitate. Using silica gel column chromatography (THF/methanol, 10:1), product 9 was synthesized and purified with a yield of 25% [68], as shown in Scheme 9.

Scheme 9. Synthesis and Purification of product 7 with a yield of 25%; (i) K2CO3, DMF, Ar; (ii) NH3, CH3ONa; (iii) Lu(CH3COO)3·H2O,1,8- diazabicyclo(5,4,0) undec-7-en (DBU), octanol, Argon atmosphere
2.5 Triple-deckers phthalocyanines synthesis
The triple deckers are a range of products, in connection with the product distribution varying based on reaction circumstances, metal type, and macrocycle substituents. One of the main goals of this research was to see if triple-decker compounds could be manufactured and connected into arrays in the same way as monomeric porphyrin building blocks could [69]. The nature of the tetrapyrrole macrocycles itself may also be used to check the optical characteristics of triple decker compounds with porphyrins, phthalocyanines [70]. The Bi2 Pc3 combination is generated using the procedure outlined without the use of iodine Bi2 Se3 + 12 o-DCB → Bi2 Pc3 +3Se 0, In a 1:12 molar ratio, powdered Bi2 Se3 (0.64 g) and o-DCB (1.54 g) were combined. And it was heated at 220 °C for 24 hours after being evacuated and sealed. The liquid o-DCB undergoes catalytic tetramerization at this temperature [71].
Bismuth (III) triple-decker phthalocyanine, Bi2Pc3, Pc=C32 H16 N82-, is obtained via the reaction of Bi2 Se3 with 1,2-dicyanobenzene at 220 °C, and the resulting crystals have a triclinic crystal structure. Each bismuth (III) ion has four N-isoindole atoms connecting it to the center Pc ring and four N-isoindole atoms connecting it to the outer Pc ring. It is possible to characterize the structure of metal(III)-phthalocyanines using the general equations M2Pc3 or MPc2 with a single monooxidized phthalocyaninato ring. The same method used to create bismuth(III) phthalocyanine complexes yields two distinct forms, but in an iodine environment is different [71], as shown in Scheme 10.
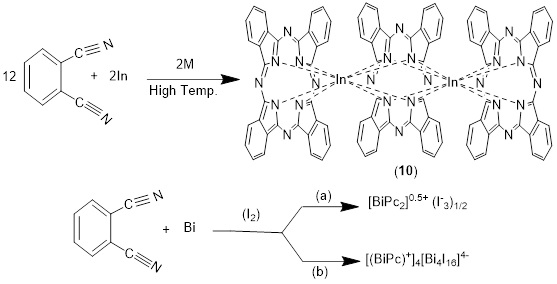
Scheme 10. Synthesis triple deckers phthalocyanines (10)
These triple-decker complexes are air-stable in the most popular solvents contained CHCl3 and CH2Cl2 and include up to eight individual one-electron redox processes [72], as shown in Scheme 11.
.jpg)
Scheme 11. Heteroleptic corrolee phthalocyannine europium triple-decker complexes Eu2[Pc(R)8]2[Cor(C1Ph)3]schematic molecular structure (11)
2.6 Synthesis of ball-type metallophthalocyanines
This type of Phthalocyanine was obtained by combining 4-nitrophthalonitrile and 9,9-bis(4-hydroxtphenyl) fluorine at high temperature using dry DMF and K2CO3, in this way [4,4-9,9-bis(4-oxyphenyl) fluorene di phthalonitrile was formed by adding CuCl2 in the presence of N2 atmosphere, ball type (9,9-tetrakis-bis (2,10,16,24)-CuPc(II) 4-oxyphenylfluorene) was discovered, as shown in Scheme 12. Conditions and reagents: i: K2CO3, DMF, N2, 24 h, rt; ii: CuCl2, N2, 360 C for compound CuCl2 [73].
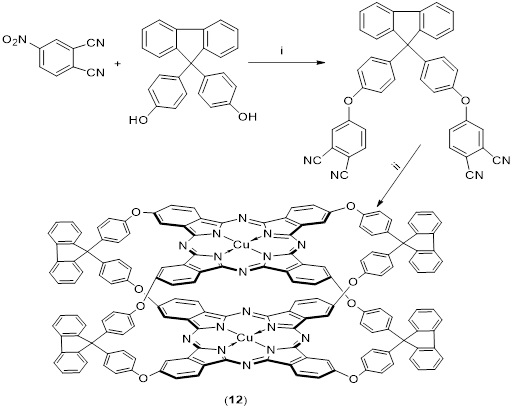
Scheme 12. Conditions and reagents: i: K2CO3, DMF, N2, 24 h, rt; ii for compound CuCl2, N2, 360 oC
2.7 Synthesis of triad phthalocyanines
One of the most beneficial cross-coupling processes is known as the Suzuki coupling reaction [74]. It is coupling has also been used to make covalently heterodyne pairs, or Pc-Pc couples, the Pc–(Pc)2 homotriad, and Pc–porphyrin heterodyads that are directly conjugated through carbon-carbon bonds [75]. The molecules are made using a simple one-step process [74], as shown in Scheme 13.

Scheme 13. Suzuki coupling conditions used to create a Pc homotriad (13)
2.8 Synthesis of polymer phthalocyanines
Hanack and colleagues produced and developed main-chain-type Pcs polymers, which depend on connecting the metal ions inside the core with proper bivalent bridges [76]. This method establishes a canonical configuration for the Pc molecules inside it, this arrangement within the so-called "shish-kebab" polymer. This method yields compounds with good solubility and up to 200 macrocyclic units [77]. As the polysiloxane backbone passes through the middle of the Pc rings, the Pc molecules are able to stack closely together, with just 0.34 nm of clearance between them. Kobayashi et al. reported a different structured phthalocyaninato-polysiloxane system, in which rod-like Pc polymers were placed into organized hexagonal channels using a sol-gel method to create organic-inorganic composites [78], as shown in Scheme 14. The development of porous organic polymers (POPs) based on pc has been swift and multifaceted. POPs are built from a wide variety of organic monomers to create their highly polymerized framework. POPs may have their molecular structures and characteristics engineered and manipulated to suit certain purposes [79].
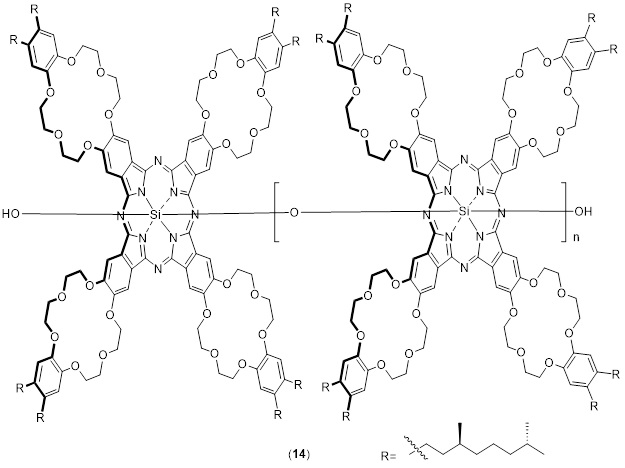
Scheme 14. Pc polymer as a main-chain polysiloxane (14)
2.9 Phthalocyanines synthesis with a trifluoroethoxy group
Because of the repulsion of the trifluoroethoxy group, it is well-documented that aggregation may be prevented using trifluoroethoxy-substituted phthalocyanines. Furthermore, fluorine's high electronegativity altered these features. As a result, as compared to non-fluorinated molecules, many fluorine-containing substances are resistant to oxidation and metabolism. In the disciplines of medicinal and agricultural chemistry, fluorine is also a vital component. It's hard to dissolve Pc in conventional organic solvents because it is very symmetrical due to its conjugate planar structure. They are chemically and thermally stable because of the strong bond between carbon and fluorine [80]. Tri fluoro ethoxy the phthalocyanines with substitutes (TFEO-Pcs) with tri fluoro ethoxy group (-OCH2CF3) presence of phthalocyanine periphery may be easily produced from trifluoroethanol, as can other fluorine-containing phthalocyanines (CF3CH2OH). To begin, with tetrafluorophthalonitrile that is readily accessible to the public (1) and combined with trifluoroethanol to make tetrakis (trifluoroethoxy)phthalonitrile (2). After that, tetramerization is used to induce TFEO-Pc as part of a broad phthalocyanine synthesis, shown in Scheme 15. Because TFEO-Pcs compounds have a significant proportion of fluorine atoms, they are very soluble in organic solvents [80].
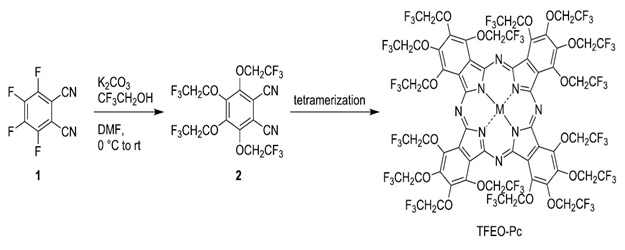
Scheme 15. Tetramerization use to induce TFEO-Pc
- Applications of Phthalocyanines
M-Pcs have a critical part in human life applications, due to the fact their various construction chemistry, electrical, with optical characteristics, and they have a wide range of applications [81,82].
Three reasons account for the majority of phthalocyanine's commercial impact: the initial characteristic is their striking and vibrant shades spanning from blue to green, coupled with their remarkable coloring strength. The second feature is a subsequent attribute that pertains to their robust chemical stability. For instance, copper phthalocyanine can sublime at an exceedingly high temperature of 5800°C and can also dissolve in pure sulfuric acid without undergoing disintegration. The third has exceptional light fastness. It's exceedingly tough to find a property combination like this [83]. This part of a research looks at how phthalocyanines have advanced as contrast agents in different varieties in the last several years [84].
3.1 Traditional applications
By far the most important is the copper derivative. It's incredibly stable and produces bright, crisp cyan hues. Because the Sort band absorption tails into the visible area some other metal phthalocyanines aren't as stable, and/or they are a duller green. As a result, copper phthalocyanines will be the focus. Copper phthalocyanines are pigments as well as dyes [85].
3.1.1 Copper phthalocyanine pigments
The majority of pigments made of phthalocyanine molecules, (copper phthalocyanine) such as CI Pigment Blue 15- can exist in several crystalline forms. The α--form is more environmentally friendly so greener, and more stable β--form are the two primary polymorphs of copper phthalocyanine. When it comes to charge generating materials for laser printers, polymorphism is critical.
Copper phthalocyanine is produced in a variety of ways, but all of them result in coarse crystal form, which cannot be used as dyes. In this way, the metastable, tiny particulate-form may be created, which is excellent for, using a variety of processes (for example, sulfuric acid precipitation or sodium chloride grinding) [85].
The effects of organic pigment's molecular structure, the kind of dispersion agent used, and the length of the ball milling process were examined. Comparing Cl Pigment Green 7 to other pigments, the results indicated that it had higher dispersion stability over time [86].
One of the most significant families of colorants among them is copper phthalocyanine, which has exceptional qualities including light resistance, tinting strength, covering power, and resistance to alkalis and acids. As previously stated, depending on the level of substitution, colors move from blue to green due to the presence of halogen substituents like chlorine and bromine, which also serve to stabilize the form. Printing inks are the most common use for the greener version of copper phthalocyanine, and CI Pigment Green 7 is a prominent example of this kind of pigment [87], as shown in Scheme 16.

Scheme 16. CI Pigment Green 7
3.1.2 Copper phthalocyanine dyes
The lower relevance when phthalocyanine pigments are compared to phthalocyanine dyes is due to the enormous size and stiffness of the molecules. It's too big to dye polyester and polyacrylonitrile synthetic fibers, and it's only useful for nylon. As a result, it is nearly solely employed to color Cotton and paper are examples of cellulosic substrates. Copper phthalocyanine derivatives are also used to make significant ink jet printers' cyan dyes. It is well knowledge that CI Direct Blue 86 is a kind of phthalocyanine cyan dye. It is the first commercially available phthalocyanine dye [83], as shown in Scheme 17.
Scheme 17. CI Direct Blue 86
3.2 High-technology applications
The unique chemical and physical features of phthalocyanines make them ideal for a variety of applications, including effective color, chemical, and thermal stability [88], and high conductivity, also used in organic light emitting devices and printers [41].
3.2.1 Ink jet printing
Over the last decade, inkjet printing has advanced dramatically [89,90], with developments in colorant importance and ink technology using a key role. Copper phthalocyanine dyes stand out as the preferred cyan dyes for nearly all inkjet printers due to their unique combination of characteristics (mentioned previously).
Early ink jet printers, which initially debuted by the end of the 1980s, had to rely on generic coloring agents that had been cleaned up to a considerably higher quality for ink jet use. CI Direct Blue 199 as shown in Scheme 18 was the cyan dye of choice at the time, and it is still commonly utilized in today's ink jet printers.
 Scheme 18. CI Direct Blue 199 (18)
Scheme 18. CI Direct Blue 199 (18)
This dye has excellent all-around qualities. However, it is particularly water-soluble in most situations [91].
Avecia's research [92] resulted in a dye with significantly improved water fastness, yet it was still far from flawless. The theory of differential solubility was applied to create this dye as shown in Scheme 19. Most ink jet inks have a pH between 7.5 and 10, making them alkaline, while the majority of paper surfaces are somewhat acidic to neutral (pH 4.5-7.0). The Carbonyl (C=O) group possesses the right pKa to present in the ink in its ionized, water-soluble state, but to transform, upon contact with the paper, into the free acid, water-insoluble form.
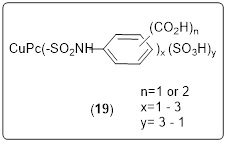
Scheme 19. Avecia dye production (19)
Ink jet printing is also starting to employ CI Pigment Blue 15, especially when great endurance is required, such as in many outdoor applications.
3.2.2 Electrophotography
Photocopying and laser printing are two common examples of electrophotography. Titanyloxy phthalocyanine type IV polymorph [93] is the best material for generating latent images in current laser printers. It has a high photoinduced discharge speed and is compatible with semiconductor infrared lasers [89]. The utilization of CI Pigment Blue 15, commonly known as copper phthalocyanine pigment, in the cyan toner is an anticipated choice. Hexadecafluoro copper phthalocyanine stands as a prominent material for facilitating electron transport within organic semiconductors [94]. Additionally, blue and green phthalocyanine pigments serve as the primary color agents employed in the fabrication of color filters for liquid crystal displays [95].
3.3 Flow-injection analysis (FIA)
The use of MPs and Pc have been clear to be useful in flow injection, sequential injection analysis, and microfluidic systems have been proven to be effective.
3.3.1 Phthalocyanine liquid chromatography flow injection analyzer (FIA)
Phthalocyanines were most likely initially utilized with the advent of FIA in 1984 for use in liquid chromatography's flow-injection analysis (FIA), phthalocyanine-containing chemically modified electrodes for electrochemical detection in LC/FI systems became commonplace [96]. The quantification of captopril, thiopronine, and penicillamine was accomplished using a combination of liquid chromatography and flow-injection analysis. This approach involved utilizing electrochemical detection in conjunction with an electrode that incorporated a conductive carbon cement (CCC) matrix. This electrode had undergone chemical modification with cobalt phthalocyanine. The procedure was extensively elucidated in the research conducted by Favaro and Fiorani [97].
3.3.2 Electrochemical methods/ phthalocyanines
Electrochemical sensors and biosensors rely heavily on Pcs and M-Pcs containing cobalt (II) phthalocyanines (CoPcs). Data and information regarding the use of Flow-Injection-Analysis (FIA) with phthalocyanine-modified electrodes make up the vast bulk of what is currently available [98].
The Pc family's significance in various applications has been firmly established, thanks to its structural adaptability and distinctive characteristics arising from the integration of diverse inorganic and organic elements into its structure [99].
3.3.3 Modified carbon-paste electrodes
The electro-oxidation of organic peroxides was facilitated using cobalt-phthalocyanine modified carbon-paste electrodes, as demonstrated by Joseph Wang and coworkers [100]. It is impossible to reverse the oxidation process. The use of a cobalt-phthalocyanine-modified carbon paste electrode for the detection and determination of amitrole in flow injection analysis demonstrates the usefulness of such electrodes as indicators for electrocatalytic amperometry measurements of triazolic herbicides like amitrole at low oxidation potentials (+0.40 V) [101].
3.3.4 Electrodes made of modified glassy carbon
The oxidation of the antioxidant tart was the subject of research conducted by Pingarrón, who examined the behavior of a nickel phthalocyanine (Ni(II)-4,9,16,23-tetraaminophthalocyanine) polymer covered glassy carbon electrode.BHA, or –butylhydroxyanisole [98].
In two papers, Shaidarova et al. changed glassy carbon electrodes. The first study used a glassy carbon electrode's electrocatalytic reaction after having a polyaniline layer deposited on it and then having copper (II) tetrasulfo phthalocyanine to determine dopamine under static circumstances and with FIA. Dopamine oxidation was mediatized using a polyaniline composite film containing copper (II) tetrasulfophthalocyanine and applied to a glassy-carbon electrode (GC) in both acidic and neutral environments [102]. In their second work, they described the electrocatalytic oxidation of sulfur amino acids and flow-injection measurements at a glassy carbon electrode modified with a nickel (II) polytetrasulfophthalocyanine screen [103].
The electrode, made of glassy carbon with copper modifications, Maria Sotomayor and colleagues [104] developed a bio-inspired sensor for monitoring sodium diclofenac (II). In recovery investigations, the usage of a sensor For pharmaceutical preparations as well as biological blood samples, the findings were remarkably similar to those obtained by HPLC [103].
3.3.5 Modified graphite electrodes
The measurement of N-acetylcysteine (NAC) in pharmaceutical formulations was done using flow injection analysis (FIA) with amperometric detection, using an ordinary pyrolytic graphite (OPG) electrode modified with cobalt phthalocyanine (CoPc). In preliminary research, cyclic voltammetry was utilized to determine the optimal conditions for NAC analysis. For the voltammetric measurements, in this technique, used a three-electrode cell with a pyrolytic carbon electrode, a platinum wire electrode, and a calomel electrode [98, 105].
3.3.6 Boron-doped diamond electrodes with modifications
To achieve highly sensitive detection of hydrogen peroxide, a modified electrode consisting of cobalt phthalocyanine on boron-doped diamond (CoPc-BDD) was developed. The process involved the photochemical transformation of the hydrogen-terminated surface of the BDD electrode using 4-vinylpyridine, resulting in the introduction of pyridine groups. Subsequently, these pyridine moieties were immobilized on the electrode surface by immersing it in a solution containing cobalt phthalocyanine. Through cyclic voltammetry, it was demonstrated that the CoPc-BDD electrode exhibited catalytic activity for the electrochemical oxidation of hydrogen peroxide. This modified electrode, referred to as CoPc-BDD, it was demonstrated as an appropriate selection for hydrogen peroxide detection when incorporated into a flow-injection analysis-electrochemical detection (FIAECD) system [106].
3.3.7 Modified screen-printed electrodes
Various M-Pcs were used to modify screen-printed carbon electrodes and use them as sensors in FIA applications. In 1997, Rippeth and colleagues released a paper detailing the development of a wall-jet flow cell equipped with a screen-printed amperometric pesticide biosensor and a complete flow-injection system. The first biosensor was made by using the technique of encapsulating the enzyme acetylcholinesterase (AChE) on a cobalt phthalocyanine screen-printed carbon electrode [107].
3.3.8 Membrane matrices made from modified poly (vinyl chloride) PVC
Two new potentiometric azide membrane sensors were documented, with one of them employing manganese(III) porphyrin [Mn(III)P] ionophores dispersed in plasticized poly(vinyl chloride) PVC matrix membranes and the other using cobalt(II) phthalocyanine [Co(II)Pc] ionophores distributed in the same way [108]. The near- and sub-Nernstian membrane sensor responses based on Mn(III)P and Co(II)Pc in batch mode were -56.3 and -48.5 mV/decade, respectively. The pH of the environment did not affect the sensors' responses, so the range of 3.9 to 6.5 was steady within 0.9 mV for at least 8 weeks. Most typical anions that are commonly connected with azide ions did not create any interference. The growth of a groundbreaking FIA automated potentiometric method for measuring selenium as well as was discussed, along with its applications in pharmaceuticals and the analysis of anodic slime [109].
3.4 Photodynamic therapy (PDT)
In general, new cancer medications and clinical trials have resulted in very minor improvements in treatment results [110]. The number of new clinically authorized medications is modest [111]. Photodynamic therapy refers to a series of cancer treatment techniques depending on the exposure to light of photosensitizers (PS) (PDT). PDT is a highly selective and less invasive anticancer treatment method [112, 113]. Photosensitizer (PS), light, and oxygen are the three necessary components [114].
Phthalocyanines (PCs) are ingested orally or intravenously, and when the photoexcitation which results in the formation of reactive oxygen species (ROS), is produced, causing irreparable damage to cancer tissue. Among the photosensitizers used in PDT, the porphyrin derivatives and Pcs family organic dyes have garnered a great deal of interest [115].
Because of its considerable Photodynamic efficacy and a notably high molar absorption coefficient (e105M-1cm-1) within the red region of the spectrum (approximately 630-730 nm), which facilitates deeper tissue penetration of the activating light [116], zinc phthalocyanine (ZnPc) emerges as a prospective category of photosensitizers in the field of Photodynamic Therapy (PDT) [117]. These macrocycles may engage with one another via attractive stacking interactions, resulting in aggregation in solution, owing to their wide flat hydrophobic aromatic surface [118].
3.5 Organometallic phthalocyanine solar cells
Organic solar cells were made, described, and characterized, employing various pc compounds including poly (CuPc), copper tetrakis(4-cumylphenoxy) phthalocyanine, copper phthalocyanine, and titanyl phthalocyanine. These phthalocyanines demonstrated absorption of light with wavelengths exceeding 500 nm. Cu-Pc is one of the most widely studied Pcs for organic solar cell applications. It has a high absorption coefficient in the visible range of the spectrum, making it efficient at capturing sunlight. CuPc can be used as an electron donor material in organic solar cells [119]. Also, the conductivity of poly (copper phthalocyanine) (PolyCuPc) is quite high [120]. Because of their low cost and simple production technique, so many attempts have been made to develop solar cells using organic semiconductors. Pcs have several advantages to solar cells [121], including resistance to heat, mild steadiness, with a high degree of visible-range and solar cells. ZnPc is another commonly used Pc in organic solar cells. It has good electron mobility, which is important for efficient charge transport within the device [122]. The developed method for fabricating organic photovoltaic cells that use PolyCuPc, copper tetrakis (4- cumylphenoxy) phthalocyanine (Tc-CuPc), (CuPc), and titanium indium phthalocyanine (TiOPc) as donors and 6,6-phenyl-C61-butyric acid methyl ester (PCBM) as acceptors [123]. The studies will provide a method for improving the photovoltaic characteristics of inverted organic solar cells by combining PCBM with Pcs [93, 124]. TiOXPc has been studied for its potential application in solar cells, particularly in organic and dye-sensitized solar cells. It is primarily used as a light-absorbing and electron-transporting material in these types of solar cells [125].
It has been observed that organic solar cells are unstable due to photo-induced O2 migration into C60 or due to the interaction of Al electrodes with oxygen and water [126]. To solve this problem, non-corrosive Au electrode solar cells have been developed lately [127].
- Typical Characterizations of Phthalocyanines
Pcs compounds have a broad variety of unique characteristics, CuPCs, and ZnPCs compounds were chosen and employed, as described earlier. The electrical and steric impacts of surrounding groups may change the nature of the aggregative interaction with a complex ion in the cavity. The concentration, temperature, and solvent type all have a role as well. Solvents that compete with the aggregative interaction will have the greatest noticeable influence on decreasing aggregation. When the concentration of PC is raised, the molecules tend to cluster together, leading to a narrowing of the Q band and a hypsochromic shift [128].
4.1 Zinc phthalocyanine derivatized with benzothiazole or carbazole
Most organic solvents are incompatible with metallophthalocyanines however, adding substituents to the ring system makes them more soluble. Both Complex A and Complex B shown in Scheme 20 were very well readily soluble in several different types of organic solvents. Pyridine exhibited a noticeable red shift in Figures 1 and 2. Since the Q-band of phthalocyanine moves toward the red end of the spectrum as the solute's refractive index increases, a red shift is seen in pyridine Table 2, Figures 1 and 2. Ground-state absorption spectra for both A and B. Figures 1 and 2 revealed the behavior of monomers, as demonstrated with a single Q band characteristic of phthalocyanines with metal ions. Pc ring HOMO-LUMO and π-π* transitions are responsible for the visible Q band (670-700 nm) in the visible spectrum [129]. Also, in the visible spectrum, unsubstituted MPcs have a strong absorption peak between 600 and 700 nm, giving it a bluish-green hue. Substituted MPcs, on the other hand, show this absorption at longer wavelengths, and it depends on the metal ion size, core modification, and metal ions [130].
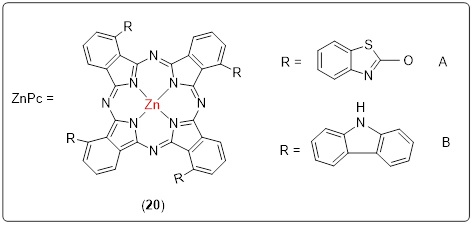
Scheme 20. Oxybenzothiazole (A), oxycarbazole (B), phthalocyanine (20)

Figure 1. Spectra of compound A absorption in various solvents

Figure 2. Spectrum of complex B absorption in various solvents
UV-Vis (DMSO)A: λmax nm (log ε); 672 (5.29), 606 (4.59), 337 (4.79). FT-IR: (υmax/ cm-1 ): 3018 (Ar–H), 1598 (C=C), C=N (1560), 1288, 1120 (C–O–C), 940 (C–S–C), 730, 714, 760, and 830 (C–H).
UV–Vis (DMSO)B: λmax nm (log ε); 700 (5.12), 630 (4.59), FT-IR: (υmax /cm-1): 3200 (NH), 3161 (Ar–H), 1576, 1077, 1110 (C–O–C), 1559 (C=N), and 1412 (C=C).
It can be shown from Table 2 that compound A absorbs most strongly between 669 and 677 nm in the Q band (in different solvents), whereas the B band absorbs across a wider range of wavelengths (336-337) owing to the overlap between the B1 and B2 bands. Q band absorption in DMSO for unsubstituted ZnPc is at 672 nm, while for benzothiazole derivatized ZnPc it is at the same wavelength. Red shift is more pronounced in the oxycarbazole substituted ZnPc complex B than in the benzothiazoles derivatized Pc or the unsubstituted ZnPc. There is evidence that phthalocyanine deforms significantly when large, central groups (like phenyl groups) overlap with smaller, peripheral groups. As a consequence, the Q band splits and red shifts. Since the oxycarbazole substituent B is more massive than the benzothiazole, it may account for the red shift seen in compound B relative to A. As may be seen in Figure 3 below 1.1×10-5 M, complex 1 complied with Beer-Lambert's law. Similar results were seen for complex B [129].
Table 2. Different solvent spectra for the compounds A and B
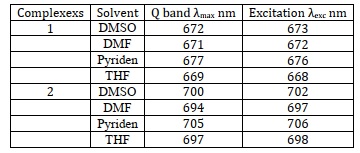

Figure 3. Complex A absorption spectra in dimethyl sulfoxide (DMSO). Place a plot of concentration vs. absorbance (demonstrating compliance with the Lambert-Beer law) here [119].
4.2 A conducting polymer and zinc phthalocyanine Zn(II)Pc
Zinc phthalocyanine Zn(II)Pc is characterized as having four quaternizedimidazolyl moieties Scheme 21 (A, B). To characterize Zn(II)Pc, 1H NMR spectra at 400 MHz were obtained using a Bruker AC-400. An Analytic JENA S 600 used a UV-Vis spectrophotometer to get the UV-Vis spectra. Zn(II)Pc (21 B) was easily resolved in a 1H NMR spectrum in Figure 4 using the polar coordinating solvent d-DMSO, with the protons in the Pc macrocycle and aromatic protons in the imidazole fragment placed in the 9.7-8.1 ppm region. The presence of methyl (N-CH3) groups may also be seen in the spectra, peaking at around 4.1 ppm.
1H-NMR (CD3OD/with drop of TFA, 400 MHz) A: δ (ppm) = 9.5-9.1(m, 6H), 9.09-8.8 (m, 2H), 8.5-8.1 (m, 8H), 7.9-7.8 (m, 4H), 2.95 (d, J = 4 Hz, 6H), and 2.88 (d, J = 4 Hz, 6H). A
1H-NMR (DMSO-d6, 400 MHz) B: δ (ppm) = 9.7-9.2 (m, 8H), 8.8-8.4(m, 8H), 8.16 (d, J = 8 Hz, 4H). 4.11 (d, J = 8 Hz, 12H), and 2.95 (s, 12H). B
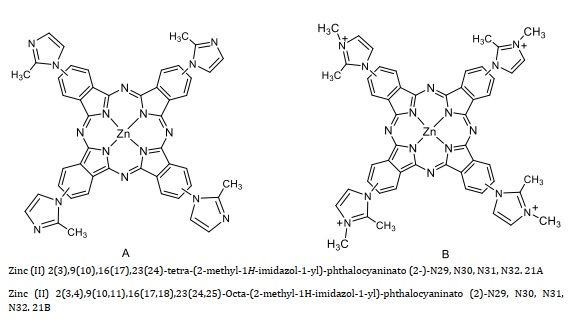
Scheme 21. Zinc phthalocyanine Zn(II)Pc (21)
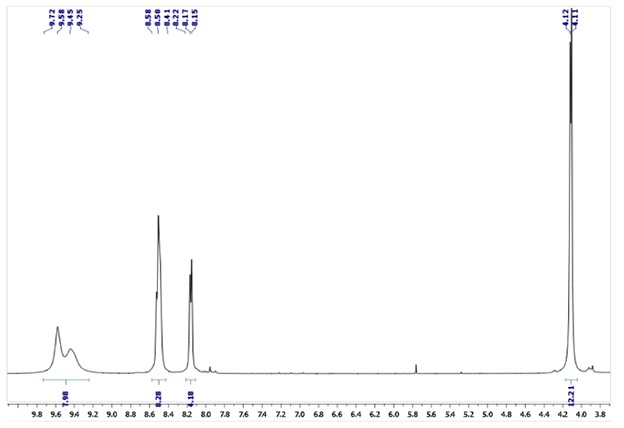
Figure 4. Zn(II)Pc (A, B).1H-NMR (DMSO-d6) spectra
Zn(II)Pc spectra in the UV-vis range, (23), the Q-band can be detected, with its center at 631 nm see Figure 5. Aggregation of Zn(II)Pc (23) is shown by a broadening of the Q band when the compound is dissolved in water.
UV-Vis (H2O): λmax(log ε) = 663 (4.66), 631 (4.84), and 337 (4.75). B
UV-Vis (DMSO): λmax, nm (log ε): 676 (5.32), 610 (4.53), and 350(4.72). A [131].
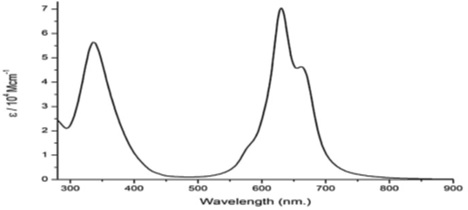
Figure 5. Zn(II)Pc UV-Vis spectra (B), in water ∼ 1 × 10-5 M [120]
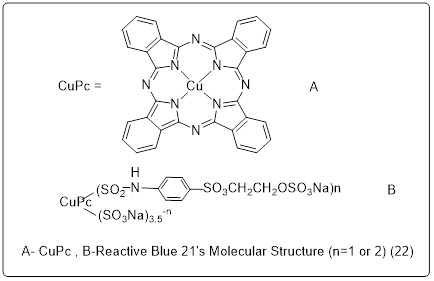
4.3 Phthalocyanine derivatives-reactive Blue 21
Scheme 22 depicts the radiation pattern for Reactive Blue 21, while Figure 6 illustrates its UV-Visible radiation pattern. The chemical contains two prominent absorption bands in its UV-Vis spectra. Electronic transitions between the π-highest (HOMO) and π*-lowest (LUMO) energy levels cause absorption in the ultraviolet (UV) range of around 300 to 400 nm (B-band). The other may be seen between 550 and 700 nm (Q- band) and is caused by π- HOMO to π* LUMO energy level transitions. The results also show that the chemical has a strong absorption between 200 and 250 nm. Absorption spectra in the UV-Visible band, from 200 to 800 nm, were captured using a Hitachi U-3310 spectrophotometer (Hitachi Co., Japan) [131].
Scheme 22. Radiation pattern for Reactive Blue 21
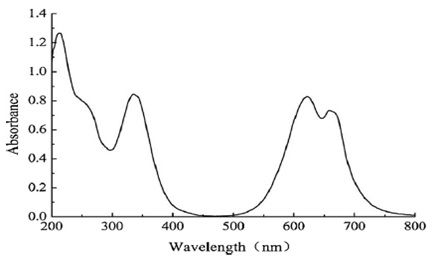
Figure 6. UV-Vis spectrum of Reactive Blue 21
The changed textiles had significantly lower reflectance than the unmodified sample over the spectrum from 550 to 700 nm. The significant absorbance of Reactive Blue 21 between 550 and 700 nm is responsible for this drop. It proves that Reactive Blue 21 can be successfully dyed into cellulose fibers. Figure 7 displays the FT-IR spectra of both the grafted and ungrafted textiles. The -C=N- group stretching vibrations and the Cu-N ligand group stretching vibrations on the modified cellulose fabric were responsible for the shoulder detected at 1595 cm-1 and 697 cm-1, respectively [132].
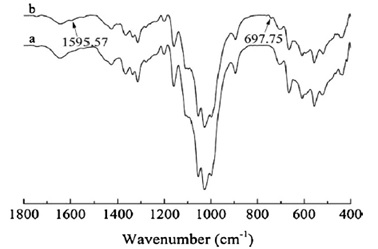
Figure 7. Comparison of the FT-IR spectra of the original cloth (a) and the altered fabric (8%) (b) [121].
4.4 Powder to thin film processing of copper phthalocyanine semiconductors
The depicted molecular structure of CuPc is shown in Figure 8 (a). Different polymorphic forms of CuPc are known to exist, with α -phase and β phase being the most often seen. As can be seen in Figure 8 (b), in CuPc molecules with the α phase, the planar molecules form an angle of approximately 65 with the b axis. Molecules of CuPc in the β-phase arrange themselves in a herringbone configuration, indicating an almost 45 angle with the b axis Figure 8 (c). Higher conductance for the α-phase crystal may be explained by the fact that the π electron overlap is greater in it than in the β-phase crystal, which results from the change in tilt angle. In Figure 8 (d), we see the XRD patterns for the current CuPc powder and films. The XRD pattern of CuPc powder showed a typical characteristic of polycrystalline containing both α and β- phases, which is a succession of peaks. CuPc films, on the other hand, show just a single diffraction peak at 6.84. This peak is congruent with that described in the kinds of literature, coming from the (200) orientation of the α-phase. In addition, the good texture of the α-phase in the CuPc films is shown by a single peak along the (200) orientation for all of the films made at various substrate temperatures (25 C, 50 C, 100 C, 150 C, and 200 C). As indicated in the inset of Figure 8 (d), the full width of half maximum (200) peaks decreases steadily as the substrate temperature rises.

Figure 8. The CuPc molecular structure (a), the layered architectures of -phase (b), phase (c), and X-ray diffraction (XRD) patterns of CuPc powders and films deposited at various temperatures for the substrate (d).
CuPc powder and film UV-Vis absorption spectra are shown in Figure 9. The absorption is caused by the overlap of molecular orbitals inside the aromatic 18 π-electron system with orbitals on the core metal atom. Also comparing the films' optical absorbance to that of CuPc powder reveals a striking difference see Figure 7, which is likely due to the phase transition. The region ultraviolet spectrum of (B band) and the visible region (Q band) (π - π * transition), CuPc films' spectra display two prominent distinctive bands. There are two primary absorption peaks inside the Q band, one at 620 nm due to the dimer and the other at 680 nm due to the monomer. Typical of α-CuPc films, two peaks can be seen in the Q band about the sample with a 25 C substrate temperature: one at 622 nm and another at 696 nm in the present films. Peaking at around 622 nm, moves toward longer wavelengths as soon as the substrate heats is raised. This peak of the Q band moves redward by 8 nm, to a wavelength of 630 nm, when the temperature of the substrate reaches 200 C. The peak originates from the oscillation of free electrons inside metal ions. It is likely that, the free electron oscillation around the Cu center is affected by the growth in particle size as the substrate temperature rises, leading to a red shift in light absorption. Q band's other peak, at 696 nm, is insensitive to changes in substrate temperature. Using data from the literature, we calculated the direct energy gap (Eg) in today's α-CuPc films. For a film made at 25 C and 200 C, the computed values for Eg are 1.525 eV and 1.609 eV, respectively. According to the stated Eg (1.5 1.7 eV), these numbers are in agreement [133].
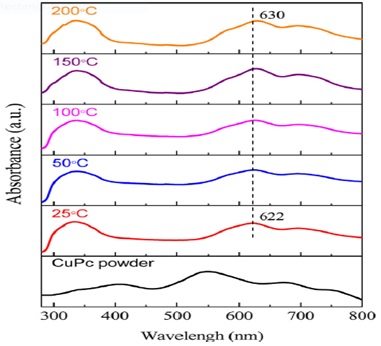
Figure 9. CuPc powder and films are made at different substrate temperatures, as shown in UV-Vis spectroscopy. The highest point of the CuPcfilms is shown by the dotted line [122].
4.5 Copper phthalocyanine derivatives
The UV-Vis Spectral comparison of new emerging materials and recycled CuPc catalysts shown in Figure 10 confirms the catalyst's stability throughout the process. There is a prominent absorption peak of about 650 nm in these spectra, and this is the Q-band, which is shared by all metallophthalocyanines and is associated with the a1u(π) eg(π*) transition. Parameters such as symmetry, center metal, etc. all of these factors contribute to the exact location of the Q band. It is worth noting that although non-metallated phthalocyanines exhibit a double-peaked Q band, the CuPc has only displayed a single-peaked Q band.

Figure 10. UV-Vis spectra of fresh and reused CuPc
In Figure 11, we see the FT-IR spectra of CuPc. The C=N and C=C vibrations in this spectra were seen at 1620 cm-1 and 1508 cm-1, respectively. Isoindole was initially seen to exhibit C-N and C-C stretching at 1332 and 1119 cm-1, respectively. Peaks at 1600 and 1470 cm-1 are associated with aromatic ring breathing vibrations; peaks at 1089 cm-1 are attributable to CH in plan deformation, while peaks at 728 cm-1 are attributable to CH out of plane deformation of the phenyl ring [134].
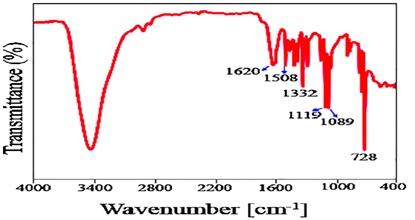
Figure 11. FT-IR spectrum of CuPc [123]
4.6 Complexes of metallophthalocyanines containing thiazole groups
The phthalocyanine system's UV-Vis absorption spectra displayed signature Q and B bands. Two prominent absorption bands were seen in the spectra of phthalocyanines. The –π-π* transition from the phthalocyanine rings of (HOMO) to its (LUMO) causes one of these to occur in the visible area between 600 and 700 nm (Q band). The other band, the B band, lies in the ultraviolet spectrum between 300 and 400 nm and results from the LUMO transition at deeper energy π levels. All of the phthalocyanines (a-c) may be best characterized by their electronic spectra. The monomeric characteristic derivatives of the tetrasubstituted compounds of phthalocyanine complexes (a and c) were indicated by a single (narrow) Qband in their electronic absorption spectra, as is typical of metallated phthalocyanine complexes. There was a characteristic B-band at 329, 344 nm and a Q band at 668, 680 nm in the UV-Vis spectra of CoPc (a) and CuPc (c) in DMF. ZnPc (b) displayed a B band at 354 nm in its UV-Vis spectrum when dissolved in DMF, as well as a strong Q band at 679 nm and a faint band at 614 nm. Compounds (a)-(c)'s UV-Vis spectra in DMF are shown in Figure 12 [128].
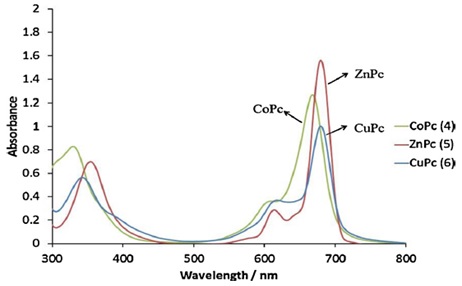
Figure 12. The spectra of electronic absorption in (DMF) show a range of 4-6. Concentration: 6.00 × 10-6 M [118]
- Conclusion
Pcs are chemical substances, organic or inorganic macromolecule compounds that were accidentally discovered more than one century ago. This study, work presents the successful results of several derivatives of Pcs with a wide range of applications that have been produced and are improving. New types of Pcs compounds show interesting development potential in electrical, electrochemical, and photosensitizers for photodynamic therapy of cancer treatments. Application and characterization of different compounds and functionalized with the synthesized variant of PCs, MPCs, exhibited monomeric behavior in solution and demonstrated increased solubility in common organic solvents. The arrival of these reviews comprising so many diverse knowledge substituents prompted us to provide this comprehensive summary of the current state and future directions of research in this area. The Pcs compounds have undergone a full structural investigation for the first time. This method has been used to examine the salient molecular interactions within the produced crystal structure. In addition, pc photochemistry and photophysics were investigated. The results showed that the produced PCs may be utilized as a photosensitizer for treating cancer and that compounds with a heavy central atom had better therapeutic effects.
Acknowledgments
To the Chemistry Department of Firat University, the authors would like to express their gratitude for providing research and technical assistance.
Orcid:
Othman Abdulrahman Hamad: https://orcid.org/0000-0001-8170-9094
Rebaz Obaid Kareem: https://orcid.org/0000-0001-6273-1309
Peshang Khdir Omer: https://orcid.org/0000-0002-6965-1843
Citation: O.A. Hamad, R.O. Kareem*, P.K. Omer, Recent Developments in Synthesize, Properties, Characterization, and Application of Phthalocyanine and Metal Phthalocyanine. J. Chem. Rev., 2024, 6(1), 39-75.


